BISOXY-LINER
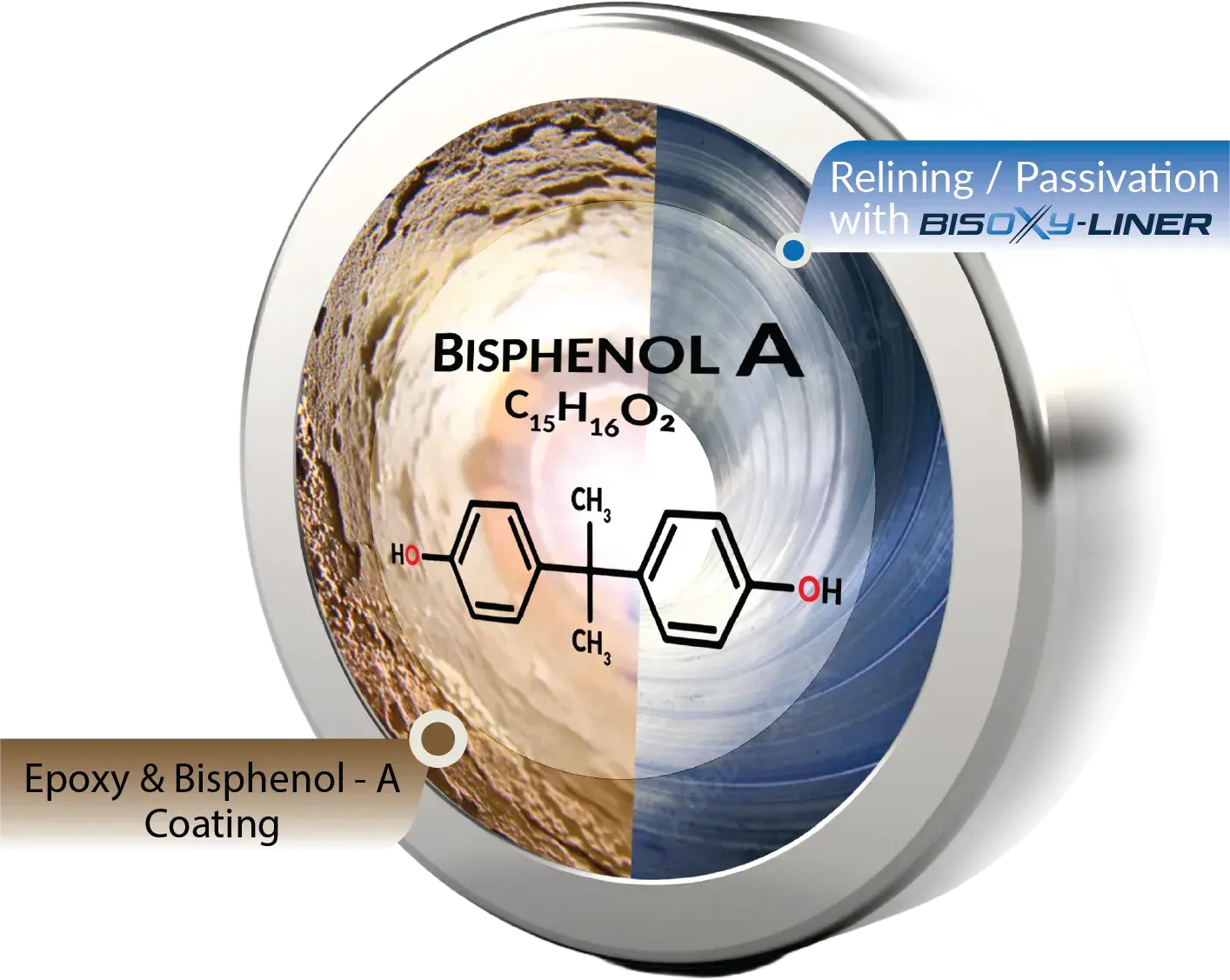
BISPHENOL OXIDATION AND PASSIVATION
BISOXY-LINER, our cutting-edge solution crafted in Germany to address BPA contamination in pipes provides a sustainable and effective alternative to traditional methods.
Our product eliminates the need for pipe replacement by employing a sophisticated process of BPA oxidation and passivation. This innovative approach ensures the reclamation of BPA-coated pipes, offering a cost-effective and environmentally friendly solution. Forged with precision in German precision, BISOXY-LINER provides an unparalleled advantage in terms of efficacy and environmental responsibility using Advanced Oxidation Process technology for generating strong reactive radical and catalyst MgO for passivation & relining of pipes. The cornerstone of BISOXY ‘s prowess lies in its ability to mitigate BPA contamination without necessitating the wholesale replacement of pipes.
Oxidation and Relining of BISPHENOL-A (BPA) coated hot and cold-water pipes instead of replacing.
FEATURES
BISOXY-LINER
WORKING PRINCIPLE
BISPHENOL A (BPA) DEGREDATION WITH BISOXY-LINER
BISOXY can eliminate very toxic and persistent organic pollutants including BISPHENOL-A. BISOXY generate very potent Reactive Oxygen Species (ROS), such as hydroxyl radicals (OH°) from powder hydrogen peroxide and sulfate radicals (SO4°) from BISOXY activation. Because this hydrogen peroxide in a powder form, it is more stable and safer for transportation and storage. SO4° has an oxidation potential of 2.5 – 3.1v + 2.7v of OH° and a long life. Additionally, BISOXY activation with Liner (MgO) can be operated at a wider pH range of 8 to 9.
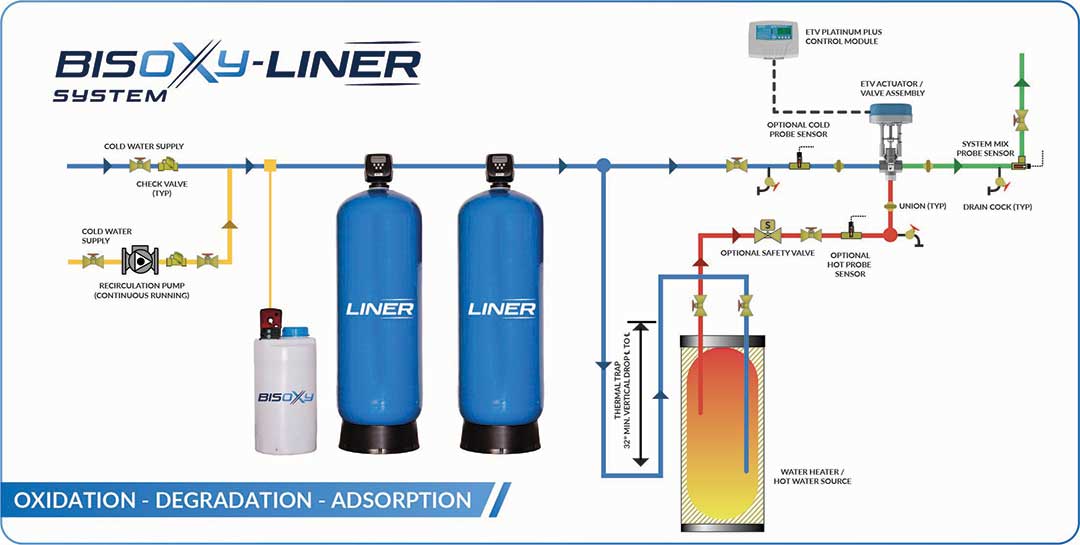
BISOXY can eliminate very toxic and persistent organic pollutants including BISPHENOL-A. BISOXY generate very potent Reactive Oxygen Species (ROS), such as hydroxyl radicals (OH°) from powder hydrogen peroxide and sulfate radicals (SO4°) from BISOXY activation. Because this hydrogen peroxide in a powder form, it is more stable and safer for transportation and storage. SO4° has an oxidation potential of 2.5 – 3.1v + 2.7v of OH° and a long life. Additionally, BISOXY activation with Liner (MgO) can be operated at a wider pH range of 8 to 9.
BISOXY–LINER is a selective oxidative dehydrogenation of BISPHENOL-A. Catalytic Degradation of Organic BISPHENOL- A with MgO.
DOWNLOAD
LINKS
FREQUENTLY ASKED
QUESTIONS
What is the main source of BISPHENOL-A (BPA)?
The major exposure of BPA is through epoxy resin coating used in food containers, Water distribution and supply network pipes, plastic bottles, microplastics, wastewater disposal, cosmetic products.
Can BISPHENOL-A (BPA) Leach out from pipes and plumbing systems in houses?
Pipes and Plumbing Systems: BPA can also enter drinking water as a result of leaching from pipes and plumbing systems that contain BPA-based materials. This is more common in older systems or those that were not designed with BPA-free materials. This leakages and BISPHENOL-A (BPA) exposure could cause a major impacts on Human Health.
How do you remove bisphenol A from water?
Most BPA treatment technologies are primarily adsorption technology, membrane technology and advanced oxidation processes. These methods are highly effective in treating BPA-contaminated water.
Using Bisoxy Liner technology, BPA could be effectively degraded when both hydrogen peroxide and a catalyst MgO were used in advanced oxidation process. BISOXY degrades BISPHENOL-A and other organic pollutants in a very fast Modus.
How do you degrade bisphenol A?
BPA could be effectively degraded when both hydrogen peroxide and a catalyst were used in the oxidation process. AOPs (Advance Oxidation Process) are the most effective and fast way for removing the target hazardous pollutants and also to get rid of Bisphenol A in water and wastewater streams.
Advance Oxidation Process (AOP) for Bisphenol A degradation?
BISOXY-LINER technology with Advanced oxidation technology would be the best suitable technology for degradation and removal of BISPHENOL-A (BPA) from water.
In wastewater and water treatment, advanced oxidation processes (AOPs) have garnered significant interest because of their potential benefits. AOPs have superior removal efficiency compared with conventional oxidation methods and are thus valuable for water purification. The generation of reactive radicals, such as hydroxyl radicals and sulphate radicals, are central to advanced oxidation process and play a crucial role in breaking down stubborn and harmful pollutants. These free radicals interact with pollutants, leading to the formation of by-products or intermediates with low molecular weights before complete mineralisation. Various AOPs are available, including electrochemical oxidation, Fenton processes, Fenton-related treatments, photocatalysis. These methods offer diverse mechanisms for promoting pollutant degradation and facilitating the purification of water and wastewater.



 Drinking Water Treatment
Drinking Water Treatment